|
Tool Batteries Explained
It seems in recent years I have been spending a lot more time in "battery management" activities than ever before. The advent of brushless DC motors and better and better batteries means more tools can be run off batteries, dispensing with extension cords and the trip hazards and hassles they represent. But with all the stories of exploding batteries and cell phones catching on fire, it might be a good time to address how we care for the rechargeable batteries in our tools.
For building code and laziness reasons, I hired electricians to wire up an addition to my house. Two guys worked pretty much all day, and at about 3:30 in the afternoon, one of the guys was having trouble drilling holes through 2 X 4s to run the last bits of wire. His drill bit was obviously dull, and his rechargeable drill was just about out of juice. His partner said, "It's 3:30, we're not getting an extension cord out now." You see, they start at 7:00AM and stop working precisely at 4:00PM.
I found this anecdote interesting because it was barely a handful of years ago that a tradesman's first order of business on a job site was stringing out extensions cords. Today, the first order of business is likely finding a place to plug in battery chargers.
To put batteries into context and understand their inner workings a bit, it is useful to think of batteries as "energy storage devices." There have been energy storage devices on earth since the beginning of time. A tree converts the energy of sunlight through photosynthesis and grows, storing energy in its trunk and limbs. Cut down a tree, stack the firewood, and you have created another energy storage device… a pile of firewood.
A dam on a river stores gravitational energy. Release the water and it can drive a turbine and generate electricity. A lake, therefore, is an energy storage device. When plants and animals died thousands of years ago, their rotting carcasses represented stored energy, much of which was converted to what we now know as oil or natural gas. Energy stored. Steam locomotives burned wood, and later coal (both representing stored energy), and converted the heat to steam that filled a boiler, and that stored energy, in turn, was used to move pistons and drive trains. A gasoline container in your garden shed is an energy storage device. When you transfer the fuel to your lawnmower, it is stored in a tank… stored energy. And if you think about it, all of these "stored energy devices" carry some degree of danger. A dam can break, a wood pile can catch fire, a gas can could explode. A battery that stores electrical energy is no different. It is an energy storage device that carries some inherent danger.
Rechargeable batteries are often called "accumulators," as they accumulate and store energy through an electrochemical reaction that is both repeatable and reversible. Contrast this with a disposable battery whose chemical reaction produces electricity, but the process is not reversible.
Once a battery is "charged," a positive active material oxidizes, producing electrons. During discharge, electrons constitute current flow. In rechargeable batteries an electrolyte serves as an internal buffer for the ion flow between the positive and negative electrodes. In some types of batteries, such as the starter battery in a conventional gasoline or diesel powered car, the electrolyte (the battery acid) is a participant in the electrochemical reaction.
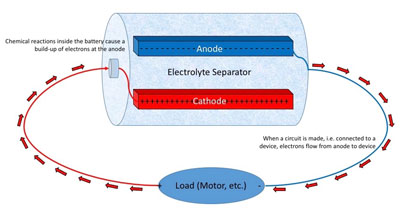
|
Figure 1 - When a load (motor, etc.) is connected to a battery,
current flows out
|
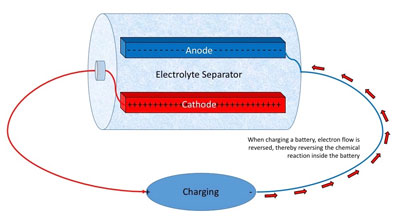
|
Figure 2 - When a charger is connected to a battery,
current flows in
|
Charging a rechargeable battery is a simple matter of applying an electrical current to the battery of higher voltage than that it produces, reversing the internal chemical processes. But here is where it gets tricky. The higher the voltage the faster the battery charges, but if it is too high, the battery can overheat and be damaged. Fancy electronics can monitor the battery closely and optimize the charging process by carefully monitoring and adjusting the in-flow voltage, the temperature of the battery, and other factors. It's all really very scientific.
The first rechargeable batteries to find widespread use were made with nickel oxide hydroxide and metallic cadmium as electrodes. These were called, appropriately, "nickel-cadmium batteries" or "NiCads." The concept was first invented in 1899 but did not come into widespread use until the 1990s. NiCad batteries have a nominal output of 1.2 volts, thus "ganging" these cells gave us the familiar 9.6 volt (8 cells X 1.2 volts), and 14.4 volt (12 cells X 1.2 volts) hand tools to which we became accustomed. Remember this point, more about this later…
Of course we also remember our old NiCad tools as heavy, taking a relatively long time to recharge, and that they were subject to what we often referred to as the "memory effect." The batteries, if not meticulously charged full, discharged fully, then recharged fully again, would quickly diminish in effectiveness.
NiCads quickly lost market share and gave way to nickel-metal hydride, or NiMH batteries. Similar in function to NiCads, the NiMH batteries were superior due to their less-toxic hydrogen-absorbing metal hydride alloy (cadmium is considered a toxic waste) and because of their enhanced electrical storage properties. A NiMH battery can have two to three times the capacity of a similar-sized NiCad battery. NiMH batteries quickly became the predominant battery in rechargeable tools.
Still though, even with the advances brought by NiMH batteries, there were problems. Rechargeable batteries "self-discharge" in storage, have limited charge-discharge cycle life, some memory effect, and the energy density available for a given size and weight of battery is limited. So, as is often the case, we benefitted from technological advances in other fields. Enter the Lithium-Ion battery, or "Li-ion."
In Li-ion batteries, lithium ions move from the negative electrode to the positive electrode during discharge, and reverse the flow during charging. These batteries offer vastly superior energy density compared to NiMH, almost undetectable memory effect, and very low self-discharge rates. Any they can be made small! Perfect for smart phones, music players, smart watches, and yes, tools. But it is these battery's "smallness" – the technology industry's obsession with miniaturization - that may be behind the fires and explosions in some smart phones.
In a lithium ion battery, the electrolyte (through which ions flow) is flammable. In addition, the electrolyte is encased in the battery under pressure. In extremely small lithium batteries, the positive and negative electrodes can be very close together, just microns apart. Even a small trace of stray metal can bridge the positive and negative electrodes causing a short, thus creating a heat source. Heat and flammable liquids are never a highly recommended combination. Carry your phone in your hip pocket? Might want to reconsider. Pressure on the lithium ion battery could deform its shape, also bridging the tiny gap between electrodes, creating a short. And if any condition exists within a Li-ion battery that is less than perfect, the very act of charging the battery may be enough to set off an irreversible chemical-heat-fire reaction… especially if the battery is damaged and left connected to a charger for longer than necessary.

|
Figure 3 - The chargers in my "charging station" have changed over
the years, but the concept stands... a power strip for all chargers that
can be turned off with a single switch.
|
Now here is the good news! We woodworkers buy tools in a relatively small market niche of what I call "hand-me-down" technology. While Apple computer develops batteries custom-shaped to its Watches and iPhones and lives on the bleeding edge of technology, they can afford to… they sell billions of devices. Yes, that is "billions" with a "b." A fantastic high-in-demand power tool might have an annual sales audience measured in the tens or hundreds of thousands… but certainly not millions, and absolutely not billions. Tool manufacturers are not developing leading edge battery technology. They are buying and repackaging battery technology from other companies. As a result, they tend to obtain products that are a little less on the "bleeding edge" of technology and more on the "proven edge." And there is nothing wrong with that.
I once heard that what we see in satellite images on our computers is four generations removed from that to which the military has access. As they increase the resolution of their imagery, so too does the "public domain" resolution increase… but we always stay about four generations of technology behind. Probably for the best; there is just no telling how some people might use high resolution pictures of their neighbors' back yards.
So the fact that we get slightly less "bleeding edge" technology in our tool batteries is just fine with me. The batteries our tools use might not be the absolute latest and greatest technology, but they are more mature technology, less likely to catch fire or explode.
Still though, even with the more "technologically proven" batteries we use, certain care should be exercised in order to make sure we get the longest service life, safest use, and most enjoyment from our investment.
-
Watch the temperature. Leaving a rechargeable tool in the sun, on a hot dashboard, or storing it on top of the radiator in your shop is probably not the best idea. Imagine that a tool battery enjoys the same temperature you do… keep it warm in the winter, but not too warm, and cool in the summer, but not too cool, and the battery will last much longer. In laboratory tests, Li-ion batteries demonstrated statistically significant shortened life and functionality at temperatures of just 86-degrees Fahrenheit!
-
Don't leave batteries on the charger overnight. You may think… you may even be pretty sure… that your charger cuts off when the battery reaches full charge, but why take a chance? Anyway, even that little green LED that shows your charger is plugged in consumes some electrical power, so why waste it? At night, remove any batteries that are on chargers and unplug the chargers. Or, better, build yourself a charging station like mine (see Figure 3).
-
At the end of each day's hard work, or in the morning before you start, inspect your tools' batteries for signs of damage. A cracked plastic casing around a battery is a warning sign. Bent, corroded, or just-plain-dirty connections should be properly corrected and/or cleaned. Especially inspect all batteries for any sign of leakage. If any electrolyte has leaked, the battery should be properly disposed of immediately.
-
Keep an occasional eye on charging batteries. You don't have to watch them like watching paint dry, but if you take a look every once in a while, and even touch the batteries to make sure they aren't overheating while on the charger, you could head off potential problems.
-
Newer batteries, especially Li-ion variations, demonstrate very little memory effect. Most of us were taught to fully discharge our old NiCad and NiMH batteries fully before charging. With Li-ion batteries, it is actually better to charge them more frequently and when they are less than fully discharged. In lab tests, a fully discharged Li-ion battery had an effective life of 300 – 500 charge-discharge cycles, but a battery discharged to just 50% and then recharged could withstand between 1,200 and 1,500 charge-discharge cycles.
-
Always use the charger intended for the battery being charged. There are some exceptions to this rule and manufacturers don't really want me to talk about this, but we will… next month.
-
If long term storage is necessary, meaning you might not use a rechargeable tool for a couple of months, store it at less than 100% charge in a human-friendly temperature range. A fully charged battery not used for a period of time will lose significant capacity.
-
Don't expect miracles. Even cutting-edge Li-ion batteries lose capacity over time and use. After 250 or so charge-discharge cycles, you may expect to see battery capacity drop by 10% to 20% or more depending on other variables.
Next month we will look a little further into the subject of tool batteries and how to get the most from our investment. We will also look at third-party battery makers and discuss the pros and cons of "going generic."
The Big Giant Bookshelf project continues, with
Part 3A
and
Part 3B
now available to view. Thanks for reading, stay safe, and keep woodworking… it truly is the key to a happy life!
Steven Johnson is retired from an almost 30-year career selling medical equipment and supplies, and now enjoys improving his shop, his skills, and his designs on a full time basis (although he says home improvement projects and furniture building have been hobbies for most of his adult life). Steven can be reached directly via email at
sjohnson@downtoearthwoodworking.com
Return to the
Wood News Online
front page
|