Understanding Lithium-ion Batteries in Portable Tools: Best Practices for Long Working Life
by W F (Rick) Howard
Philadelphia, PA
Click on any picture to see a larger version.
There's a lot of hype about lithium-ion batteries (LIBs), mixed in with old wives' tales and just plain misinformation. But with at least 10 major tool manufacturers using LIBs, they must have something going for them. Introduced about a decade ago, LIB packs were touted as the upgrade for hardware utilizing nickel-cadmium (NiCad) cells, and with good reason: LIBs with equivalent energy output have half the weight, twice the working life (1000+ charge-discharge cycles), and 10X the shelf life of NiCads. Technology has its price, however, and understandably, we all want the most for our dollar, so how do we nurture LIB-powered tools to optimize performance, especially longevity?
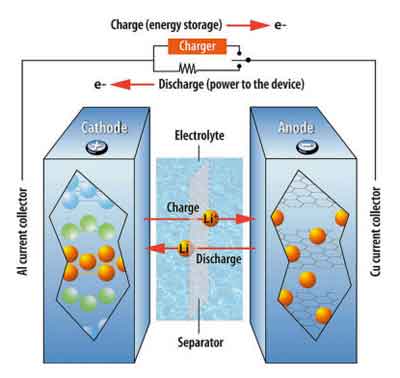
Let's first define how a Li-ion cell (in the industry, a battery or pack contains multiple cells) works, to better understand why certain actions are positives or negatives, but without delving too deeply into the battery chemistry. Figure 1 (further down below) shows how Li ions and electrons move through the cell and circuitry, respectively. The cathode is the lithium source, containing a lithiated metal oxide or phosphate that will give up Li ions and electrons when charged. The electrolyte is a salt solution with organic solvents that allows ionic passage but will not conduct electricity, and the anode is graphite, which will store lithium until the cell is used (discharged). In between the electrodes is a porous film (separator) that keeps the anode and cathode at arm's length to prevent electrical shorts.
Let me state unequivocally (and by definition), there is no lithium metal in a Li-ion battery, regardless of what the sales clerk at the Big Box store says. Lithium is, of course, a very reactive element, and will cause fire and/or explosion when exposed to moisture. The lithium ions in the cathode material are chemically bound and non-reactive; held in the graphite anode, lithium reactivity is reduced to slow bubbling when immersed in water. This doesn't mean that you can't abuse the battery until an "event" occurs: this is industry-speak for flames and loud noises, and is addressed below.
There are two factors guaranteed to shorten LIB life: water and heat. Cell manufacturers go to great lengths to minimize exposure to moisture during assembly: there are fewer than 100 parts per million water in the cell chemicals, so not to worry. Heat control is the responsibility of the user, however. This is important because every battery, regardless of chemistry, undergoes some degradation with each charge-discharge cycle. For LIBs operating at room temperature, this loss of energy output is usually less than 0.1% per cycle. But there are 10-15 different chemicals in the electrolyte, some less tolerant of high voltage environments than others, and the reaction of these species with the cathode material is the primary cause of capacity (energy) fade. Raising the temperature 20 degrees Farenheit accelerates such reactions 2-4X: degradation products build up on the anode and prevent lithium transport, and energy yields (run time) can decline as much as 1% per cycle.
|
Figure 1. Li-ion battery schematic,
showing ion and electron flows
during charge and discharge.
From J Donovan,
www.driveforinnovation.com
, 2011.
|
With the basic chemistry in hand, let's apply first principles to the safe operation and extended working life of LIBs. Working in hot weather can obviously reduce day-to-day run time fairly quickly. Just keeping the equipment out of the sun will help, and leaving hardware in a closed vehicle, where the interior temperature can reach 160 degrees Farenheit, is definitely a no-no. Over-burdening the tool will also cause heating: using a 12V or 14.4V battery pack to cut 10x6 beams or seat lag screws is outside their performance realm. Unless you are strictly a hobbyist with small projects, you're much better off spending the extra $50-100 for hardware with an 18V pack. Some manufacturers – Bosch, Panasonic, and Hitachi, among others – have sensors that automatically shut off the unit if it gets too hot, and DeWalt's 20V packs incorporate a more heat-resistant phosphate cathode material.
Another action that shortens cell life, possibly resulting in an "event," is over-charging. Battery management systems are designed to stop cell charging when the voltage reaches a preset limit (4.0 or 4.2V, depending on cell chemistry). The controlling circuitry is not as robust as the individual cells, and rough handling of the tool can damage the printed circuits, thus removing the upper voltage safety limit. This results in cell over-heating and makes the cathode susceptible to losing molecular oxygen. To summarize: an overcharged cell is hot, has a fuel source (the electrolyte solvents), oxygen, and is in a sealed container. That's a fairly accurate description of a grenade – the only difference is size. Bottom line? Don't drop the hardware.
A more subtle type of over-charging is a habit we all have – putting the unit in the charger at the end of the day so it will be full of electrons the next time we need it. The chances of an "event" are minimal, but a fully charged cell contains cathode material in its most reactive state which will more readily chew on the electrolyte solvents. Better practice is to leave the pack in its (partially) discharged state after work, which is easier on the organics, and recharge the unit 6-12 hours before its next use. And if a fully-charged unit is left in a hot location, you have everything working against you: run times will get shorter and shorter. A fine point: do your recharging in a cool, but not cold, location. The electrolyte will freeze at about -20 degrees Farenheit and stop cell operation, while charging or discharging at temperatures below 40 degrees Farenheit will stress the cell chemistry.
What if you get fumblitis and drop the tool or battery? If it lands in water, remove the pack and let it dry for several days, then recharge for 15 minutes and verify it still works (probably); if not, you need a replacement. If the impact causes cell rupture and an electrolyte leak, do NOT pick up the unit with bare hands – the liquid will react with body moisture, creating acid and leaving you with a chemical burn. Put on a pair of disposable rubber gloves, take the pack out of the tool, and leave it in a pail of water overnight. After rinsing, the gloves go in the trash, but don't forget to recycle the LIBs.
Briefly, the way to safely stretch the working life of a lithium-ion battery has the same recipe as for your own longevity: don't get overheated, don't bite off more than you can chew, take regular breaks to recharge, and keep your powder dry.
W F (Rick) Howard is a 20+ year veteran of the Li-ion industry and an avid amateur woodworker in the Philadelphia area. He can be contacted at
rikhoward@aol.com
.
Return to
Wood News
front page

